
On the morning of April 5, at the morning conference offered by President Andrés Manuel López Obrador (AMLO), the head of the Undersecretary for Prevention and Health Promotion (SPPS) Hugo López Gatell, called on the Mexican population to take part in Clinical Phase 2 of Reinforcement of the Mexican vaccine against COVID-19 Homeland.
The undersecretary also reported that the research carried out for the development of this vaccine has a significant and positive progress, especially in terms of its results, which he described as very promising for a vaccine that generates high levels of immunity in a short time. Even, he said, “it is showing to be superior” to some of the vaccines that were purchased and used in Mexico.
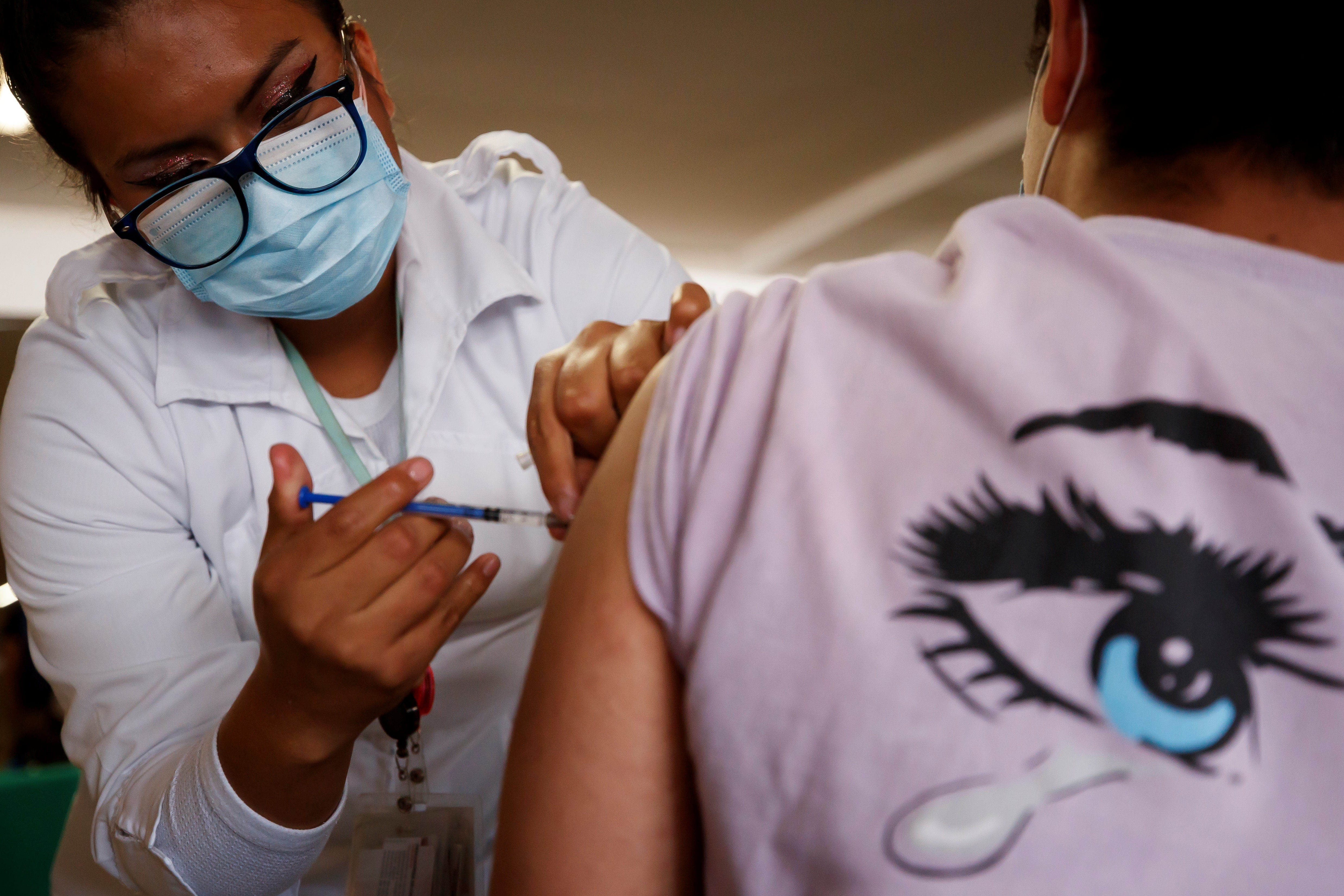
The invitation is extended to participate in the Phase 2 clinical study of Reinforcement, currently carried out with the aim of proving its effectiveness as a reinforcement for those who already have a complete vaccination schedule. In this way, the volunteers of the process, instead of applying the conventional dose (Astra Zeneca, Sputnik, Pfizer, etc.), will be given the Patria vaccine.
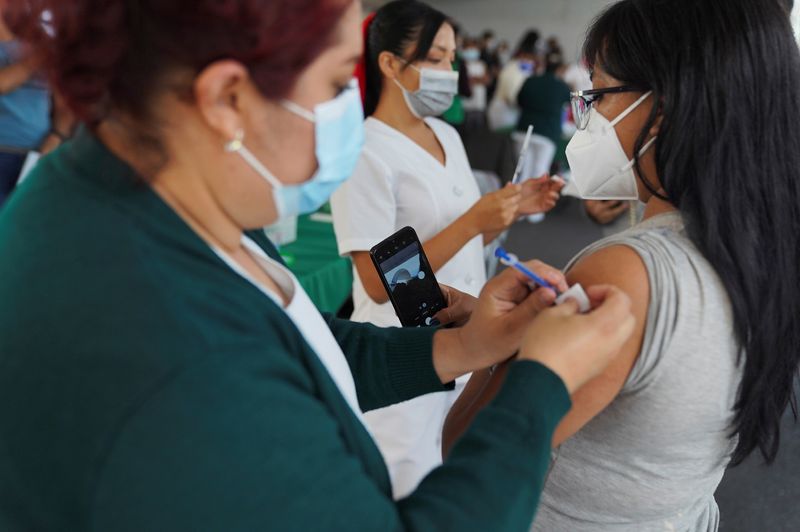
López Gatell commented that for people who participate there will be benefits such as monitoring their general health status and periodic diagnosis of COVID-19 in addition to the evaluation of vaccine immunity, this necessarily for a whole year. The registration, he said, can be done through the page of the National Council for Science and Technology (Conacyt): https://patria.conacyt.mx
The main requirements are to live in Mexico City; be 18 years of age or older; have received, at least 4 months before, a complete vaccination schedule of any COVID-19 vaccine; not having had respiratory diseases during the last 21 days; and not being pregnant or breast-feeding.
According to the authorities, Phase 1 demonstrated that the Patria vaccine induces the production of antibodies and the cellular immune response to COVID-19. Similarly, it has successfully passed the preclinical tests in living beings and the Phase 1 clinical trial in humans, which were conducted between 2020 and 2021, which were monitored and certified by the Federal Commission for the Protection against Health Risks (Cofepris).
The scientific results obtained are very promising and suggest that the development of the Patria vaccine is potentially safe and protects against the SARS-CoV-2 virus.
In 2021, President López Obrador assured that he had authorized 180 million pesos to Conacyt to continue testing for the creation of the Mexican vaccine and asserted that would be ready for implementation by the end of that year.
However, in September of that year, Marcelo Ebrard, head of the Ministry of Foreign Affairs (SRE), reported that the Mexican biologist was about to conclude its phase 1 clinical trial, so it would not be implemented in December as planned, but, in accordance with the schedule set by Conacyt, for that At the moment, the development of Patria should be about to conclude Phase 3 of clinical studies, he said.
According to the National Daily Technical Report on COVID-19, in Mexico to this day there have been confirmed 5,666,921 total cases and 323,235 total deaths from coronavirus. As of today's information cut, 7,616 active cases have been registered in Mexico.
KEEP READING:
